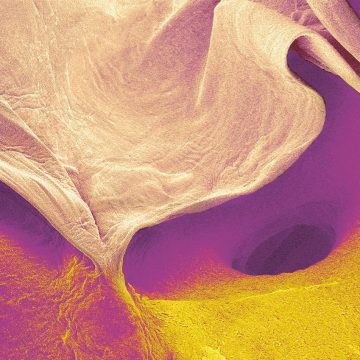
Beyond repair
As the body’s raw materials, stem cells have almost unimaginable potential to repair and regenerate. A new Cambridge Institute may just have the key to unlock that potential.
Stem cells, our body’s raw materials, are the ultimate shapeshifters. With their extraordinary ability to develop into any type of cell in the body, their potential is mind-boggling. But unlocking that potential has long been something of a holy grail for biological, clinical and physical scientists.
Now, the Wellcome-MRC Cambridge Stem Cell Institute is breaking new ground in its quest for human repair and regeneration, starting at the very essence of our being – the heart. “There are a million people who have heart failure in the UK and the current treatment regime reduces the load on the heart but can’t replace the lost muscle,” says Professor Sanjay Sinha, British Heart Foundation Senior Research Fellow and a Professor in Cardiovascular Regenerative Medicine. “The only real treatment is a heart transplant – but we only do 200 of those a year in the UK,” he says.
Unlocking the potential of stem cells has long been something of a holy grail for biological, clinical and physical scientists
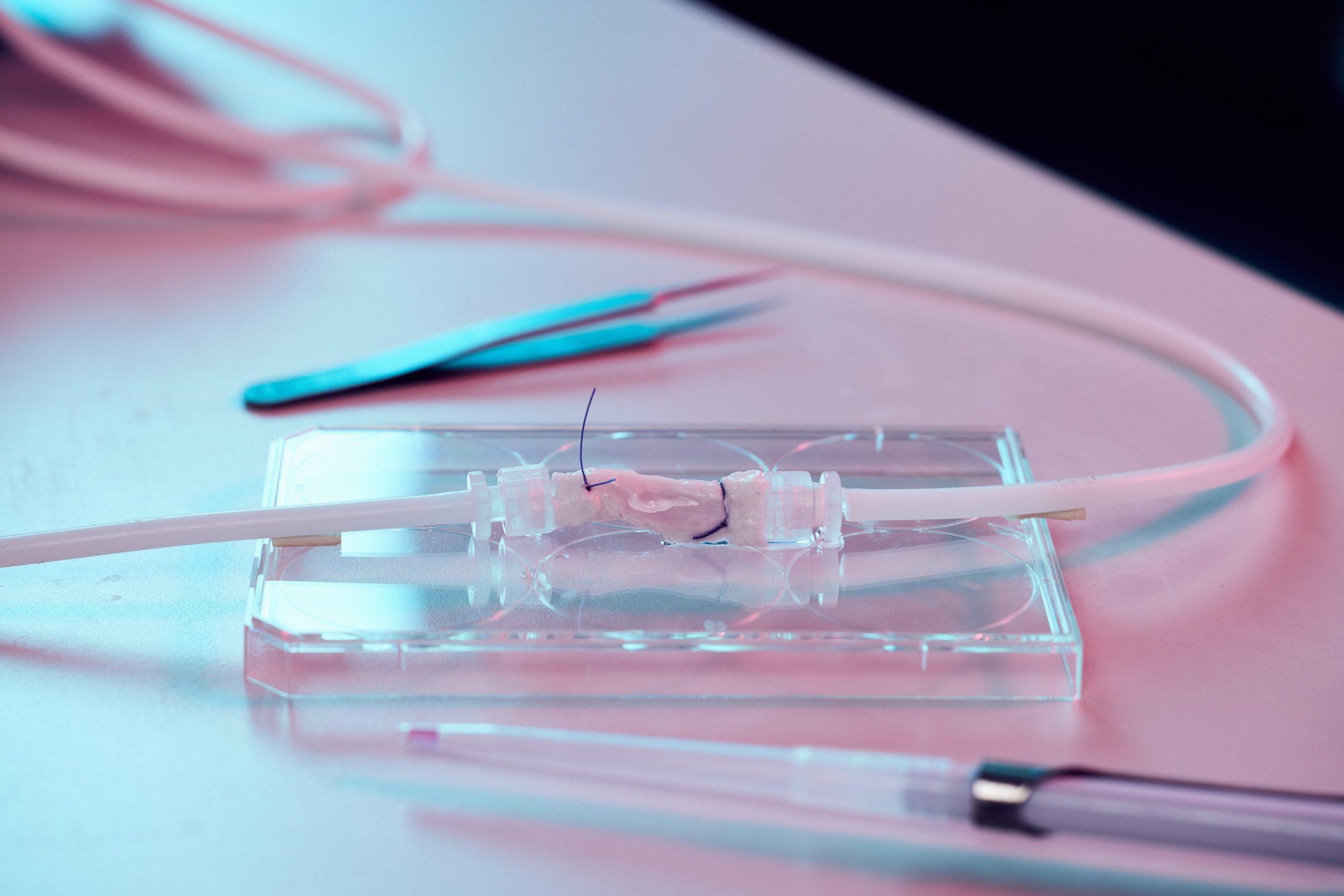
Sinha’s team is working on turning stem cells into the types of cell that are usually found in the heart: cardiomyocytes, or contracting heart muscle cells, and the epicardium, a thin layer of cells found on the outside of the heart that is vital to its development and repair. The researchers use collagen scaffolds, built by Professor Ruth Cameron and Professor Serena Best of the Cambridge Centre for Medical Materials, which are populated with a combination of heart cells and epicardial cells to create fingernail-sized patches of new material.
The cells then do something amazing. “Normally when you look at cells under a microscope, they just sit there. But these heart patches start beating. The first time I saw it, I almost fell over,” says Sinha.
“We are now carrying out studies to see if this patch works and, if it does, we will scale-up from the size of a fingernail to something the size of the palm of your hand. If that works, we could then give that to a surgeon to stitch over the damaged part of the heart.”


2) Professor Sanjay Sinha: Professor Sinha is a British Heart Foundation Senior Research Fellow and a Professor in Cardiovascular Regenerative Medicine.
3) Sinha Group: The lab’s overall aim is to develop new treatments for vascular diseases using a stem cell-based approach.
The first human trials could be within just five years. “We have shown in models that the cells work. We are currently trying to find out if patches work best and, if they do, we can move quite quickly.” What could potentially follow sounds like science fiction – 3D-printing a completely new heart to order and populating it with heart cells derived from stem cells. That is further away but still achievable, Sinha believes.
Much of the research at the Institute focuses on deciphering and mapping how the development of stem cells happens – something that’s particularly relevant to the eight million people in the UK who suffer from osteoarthritis, a condition that often leads to joint replacement. Replacement joint surgery has been described in no less a publication than The Lancet as the “operation of the century”, but treatments for the next century, if they target early disease, might not require the same level of intervention as a metal hip or knee replacement now.
What could potentially follow sounds like science fiction – 3D-printing a completely new heart to order
One of the most interesting areas of focus is the mesenchymal stromal cell (MSC), a cell that can be isolated from various areas of the body. Andrew McCaskie is Professor of Orthopaedic Surgery and Head of the Department of Surgery, and is leading the charge on MSCs. Previously described as stem cells, “these cells are multipotent – they can differentiate into different types of cell – and are easily accessible,” he says, but in fact appear to be important in inflammation by immunomodulation (therapeutic intervention intended to modify the immune response). “In terms of translation to clinic,” he adds, “every adult patient has cells like these in their bone marrow and fat, and a surgeon can easily extract them.”
McCaskie’s team is looking at how such cells can be used to repair cartilage together with a technique called microfracture. This involves a surgeon creating small holes in the underlying bone which brings about cartilage repair – not hyaline cartilage like in the undamaged joint, but fibrocartilage. They are looking to see if MSCs can influence the inflammatory response to improve the quality of the resulting cartilage. “We know these cells have been trialled for use in other conditions,” says McCaskie. “During the Covid pandemic, for example, we were able to get regulatory approval to manufacture MSCs to examine their impact on inflammatory response. We now aim to get approval to evaluate MSCs in osteoarthritis, using the same type of cell and manufacturing process.”
As the population ages, quality of life grows ever more into focus – the research being carried out by McCaskie’s team will be crucial. “People want to live their life well,” he says, and that’s exactly the sentiment driving Dr Fotios Sampaziotis and his team, looking at how advances in the use of stem cells may also be able to help improve outcomes from liver disease.


5) Dr Fotios Sampaziotis: Dr Sampaziotis is a UK Research and Innovation Future Leaders Fellow.
6) Sampaziotis Group: Research combines cholangiocyte biology, bioengineering and regenerative medicine to develop new therapies for biliary disease.
Currently, the only treatment for many with liver disease is a transplant, but even then, up to one in five see a return of the disease in the transplanted liver. A major source of the problem is diseased and damaged bile ducts, and one of the most exciting research breakthroughs the team has made is to be able to grow human bile duct cells in a dish and use them to create the first-ever working bile ducts in the lab. “We were convinced our ducts were working when we transplanted them into mice, successfully replaced the animals’ own ducts, and the mice recovered from the operation and survived long-term without any issues. We have now upscaled this to human size and we are trying to gain regulatory approval, for first-in-human studies” says Sampaziotis.
Their work is divided into two areas: growing cells in a dish and using them to test new drug treatments; and using stem cells to create organs or replacement organ parts. And one of the problems the team is trying to solve is the growing demand for healthy organs. “When disease gets to the terminal stage, your organ is completely damaged; the only way forward is to give you a new organ. There has to be a donor and it has to be a healthy donor. There is a huge imbalance between supply and demand and, unfortunately, a small percentage of people die on the waiting list while waiting for an organ to become available. If we could have ready-made organs in the lab it would solve all these problems.”
“From research over the past 15 years, we know that people with MS have damaged myelin sheaths, and stem cells in the brain respond to this”
The team is also looking at hybrid treatments. When you remove the liver from the body, the blood and oxygen supply to the organ is temporarily cut off. The tiny bile ducts in the liver are extremely sensitive to lack of blood and oxygen and often get destroyed during this time. At Addenbrooke’s, Sampaziotis is using a perfusion machine to circulate warm, oxygenated blood, which minimises small duct damage and buys time for transplanting these organs. Despite these advances, small duct damage remains the leading cause for not being able to use a liver graft, which occurs to half of all livers offered for transplantation.
“We recently had four damaged livers from Addenbrooke’s, which we had to discard due to small duct damage. So we took those organs, and tried to use our cells to repair the damage. We injected our cells directly into damaged ducts and they started regenerating them. By the end of the experiment the damage had been corrected. This means that for the first time we can use cell therapy to recover and repair an organ that would normally be discarded.”
In the next couple of years, he aims to see whether these enhanced organs can be transplanted into patients. “Finding a way to combine transplantation with regenerative therapy to create an increased pool of available organs would be a gamechanger.”



8) Dr Thóra Káradóttir: Dr Káradóttir is a co-lead of the MS Society Cambridge Centre for Myelin Repair.
9) Káradóttir Group: The lab’s interests cover neurotransmitter signalling to oligodendrocytes and their progenitor cells, in both health and disease.
A little known fact is that the nervous system also features stem cells that repair and regenerate a fundamentally crucial part of our bodies, myelin. This insulating layer forms a sheath around our bodies’ nerves, including those in the brain and spinal cord, which allows electrical impulses to transmit quickly and efficiently along the nerve cells. If myelin is damaged, these impulses slow down, something prevalent in multiple sclerosis (MS) patients.
Professor Ragnhildur Thóra Káradóttir, Director of the MS Society Cambridge Centre for Myelin Repair, explains that establishing a Centre around myelin repair in Cambridge has helped design new drugs that help to prevent the immune system from attacking the brain. The focus now has moved from immune therapy to stem cell therapies focused on repairing the myelin itself.
“From research over the past 15 years, we know that people with MS have damaged myelin sheaths, and stem cells in the brain respond to this,” she says. “We have made huge progress in the last decade. In recent clinical trials we have seen regeneration of the cells. It is a proof of concept that this can happen. It is not necessarily going to cure the disease, but if you get the diagnosis it will no longer be the Sword of Damocles.”
Across the sphere of its work, the Cambridge Stem Cell Institute offers a tantalising future where people who suffer from liver disease, osteoarthritis, multiple sclerosis and even heart failure could go to hospital and have their organs fixed using their own cells, or even replaced completely with a new organ created using a 3D printer. Working with these chameleon-like building block cells, they are turning one of life’s miracles into something extraordinarily real.
The Stem Cell Institute is looking for partners and supports. To find out more, please contact Dr Cornelius Riethdorf: Cornelius.Riethdorf@admin.cam.ac.uk