A personal approach to breast cancer treatment based on genomics
When Catharine Scott was diagnosed with stage four breast cancer, the options seemed grim. But then she was offered another way forward. The Precision Breast Cancer Institute, part of Cambridge’s new cancer hospital, is pioneering a new approach to research, care and treatment.
Catharine Scott was on holiday when she first felt the pain near her ribs. She assumed that she’d pulled a muscle in the gym or lifting a suitcase. But when she got back home, it was still there. She went to her GP, who suggested it would be wise to investigate further, and referred her to the Cambridge Breast Unit.
That was Friday. Over the weekend, Scott, 51, wasn’t particularly worried: there was no history of breast cancer in her family. On Monday she got a call from the unit, asking her to come in on the following Friday. Exactly a week after she went to her GP, she underwent a mammogram, an ultrasound and thorough examination.
Then they took me and my husband into a room and explained to us that they had seen a couple of things they were worried about
“Then they took me and my husband into a room and explained to us that they had seen a couple of things they were worried about, so they also wanted to give me a biopsy that same day to analyse the breast tissue,” Scott remembers. “I think they knew it was serious. They made an appointment for me to come back the following Friday to get the results.”
Scott’s instincts were right. Her consultant told her that she had a four-centimetre long, stage three to four tumour in her right breast. She would need chemotherapy, possibly radiotherapy and possibly a mastectomy. But unlike thousands of other women in a similar position, Scott was also offered another option. Would she take part in a trial led by the Precision Breast Cancer Institute (PBCI)?
The CRUK Cambridge Centre Breast Cancer Programme in the PBCI brings breast cancer patients into trials of cutting edge treatments and world-leading genetic research. It aims to develop personalised treatment plans based on pioneering genome sequencing activity, which determines a patient’s entire DNA and RNA profile more quickly and allows for personalised treatment of breast cancer, using new drugs. Under the NHS’s current treatment guidelines, only a few specific types of cancers are sequenced at all.

“When you have real-time information about the patient’s genome, alongside the patient being able to access novel treatments, you can then bring these two pieces of information together,” says Professor Jean Abraham, director of the PBCI and co-director of the Breast Cancer Programme. “Then you can ask: does a patient with this kind of molecular profile respond as well to that treatment as a patient with another kind of molecular profile. You can think in detail about the biology underlying who responds and why they respond.”
Scott was eligible for a new study: the Personalised Breast Cancer Programme. This research aimed to demonstrate that the genome of her tumour could be sequenced within 12 weeks, much quicker than usual. Those results would then be used to ensure she was on the right treatment programme, to identify any genetic markers which might make her more susceptible to side effects, and to inform her of any genetic markers that could increase the risk of breast cancer in her children.
Patients are helping themselves – and also investing in the future for tomorrow’s breast cancer patients
In addition, Scott was offered the chance to enrol in a clinical trial called PARTNER. The trial involved being randomly allocated to chemotherapy plus a drug called Olaparib, a drug developed at Cambridge by Professor Steve Jackson and his team. “At first, I was concerned that I would have to choose either standard treatment or going on the trial,” says Scott, “but Professor Abraham explained that I could have the experimental treatment along with the standard treatment.”
Every cancer case which comes through the Cambridge Breast Unit is discussed by a multidisciplinary team, which creates a treatment plan. Sitting in that room is a researcher from the PBCI, who identifies patients who might be eligible for a particular trial. If the patient agrees to participate, the samples they provide are used in the Centre’s own research and, with their permission, in laboratories studying breast cancer all over Cambridge, and with collaborators in the UK and around the world.
“I consider myself to be in a very privileged position to receive this consent,” says Abraham. “Patients are helping themselves, but they are also investing in the future for tomorrow’s breast cancer patients.”

Happily, Scott’s analysis found that she was on the right treatment plan, and that she didn’t have any genetic markers for hereditary breast cancer. But this personalisation of treatment, Abraham points out, is about far more than getting the right drugs. Another participant in the programme was found to have the genetic risk factors for a hereditary cancer. These would not have been picked up using the NHS’s current standard of care criteria.
“That means, in practice, we could see that she was on the right treatment,” says Abraham. “We could look at the sequencing to see if there are certain drugs she might react badly to. Surgery can also change depending on the type of cancer. But we could also notify her family to see if they wanted to see a genetic counsellor and consider getting themselves tested. If any of those family members are affected, they can undergo more frequent screenings. This patient has now been cancer-free for four and a half years – were she to relapse, we already know what our first line of treatment would be.”
In the lab, Professor Jason Carroll, co-director with Abraham of the Breast Cancer Programme, is also using the knowledge gained from research participants such as Scott to answer big questions about what causes breast cancer. His work focuses on understanding how estrogen receptor proteins that are present in every woman’s body “switch on” the process that causes cells to grow – a normal process necessary for development. But sometimes they don’t switch off: cells keep growing and cancer is the result.
The only way we can make treatments better is by doing this kind of research. Otherwise, we just stand still
Using next-generation sequencing technologies capable of analysing vast amounts of DNA, the Carroll Lab has identified where the estrogen receptor protein sits in relation to the genes it switches on. “We assumed the protein would just sit in front of the genes it switches on,” says Carroll. “But Mother Nature isn’t that simple!” They found that, in fact, estrogen receptors are relatively far from the genetic switches they use, and actually move around the genome. “And this means they’re on different switches, and the protein is switching on different genes, which will culminate in an increased or decreased response to treatment. Ultimately, that affects the clinical outcome of that woman.”
Carroll and his team are now looking further into how estrogen receptors work. “Think of the receptors like light switches,” he explains. “The DNA sequencing enables us to see where the light switches are in the room. Once we know where they are, we can take them off the wall and look at the wiring. When we did this, we found two key proteins which the estrogen receptor has to work with, and which we are now investigating – one in particular, FOXA1, has become a particular focus in our lab. The sequencing allowed us to take a completely unbiased approach and let the biology tell us where we should be looking. It taught us about long-distance switches, it found these key new proteins, and it taught us things we can use to identify and classify patients.”

Halfway through her first cycle of chemotherapy, Scott had an MRI scan that found her tumour had reduced significantly. “At the beginning of the trial, Abraham could feel the lump easily, but she started having trouble finding it by then,” says Scott. By the time she finished chemotherapy, she no longer needed a mastectomy and was given a lumpectomy instead, followed by 16 sessions of radiotherapy. That was in May 2017. Annual mammograms since have shown that she is still clear of cancer. “I’m so glad I got involved in the trial,” she says. “After all, the only way we can make treatments better is by doing this kind of research. Otherwise, we just stand still.”
Abraham and her team are now focusing on the fine details of how genome sequencing could drive better breast cancer treatment. “The genome sequencing project initially asked if we could deliver whole-genome sequencing in a timeframe which is clinically useful. The short answer is yes. So the next question is: what kind of difference does having those results in real time make?”
And all this work will come together in the new Cambridge Cancer Research Hospital, where the PBCI’s ground-breaking integrated data methods will be used as a template to analyse and treat other cancers. Abraham is looking forward to bringing together many different strands – genomics, imaging, pathology samples, clinical research – to understand their value and importance in the treatment decision-making process. “The Cambridge Cancer Research Hospital will bring together those pieces,” she says. “But we are building those pathways now. When we step into the new hospital, we’re not just replicating what we did in the old world. We’re doing something new and novel.”

Cambridge Cancer Research Hospital
Professor Richard Gilbertson, Director, Cancer Research UK Cambridge Centre
“The mission of the new Cambridge Cancer Research Hospital is to end death and disease from cancer through research, treatment and education.
The PBCI has shown us how to think about integrated cancer medicine: all these techniques are equally applicable to any cancer. We will diagnose and intervene as soon as possible, using AI and machine learning to bring together patient information and use it to make the best clinically informed decision, deploying the next generation of therapies.
The hospital will bring together the very best of Cambridge’s expertise across sectors – from medicine to physics to engineering – under one roof. But you won’t have to live in Cambridge to benefit, as we are developing these platforms to be deployed across the NHS – and across the world.
We have had great success since the 1970s with cancer. But that’s largely been through using existing treatments and looking after patients better – and investment there is diminishing. We need massive breakthroughs, not incremental changes. To do this we need a complete roadmap of the patient. The new hospital will provide that map – an A to Z of every patient’s disease.”
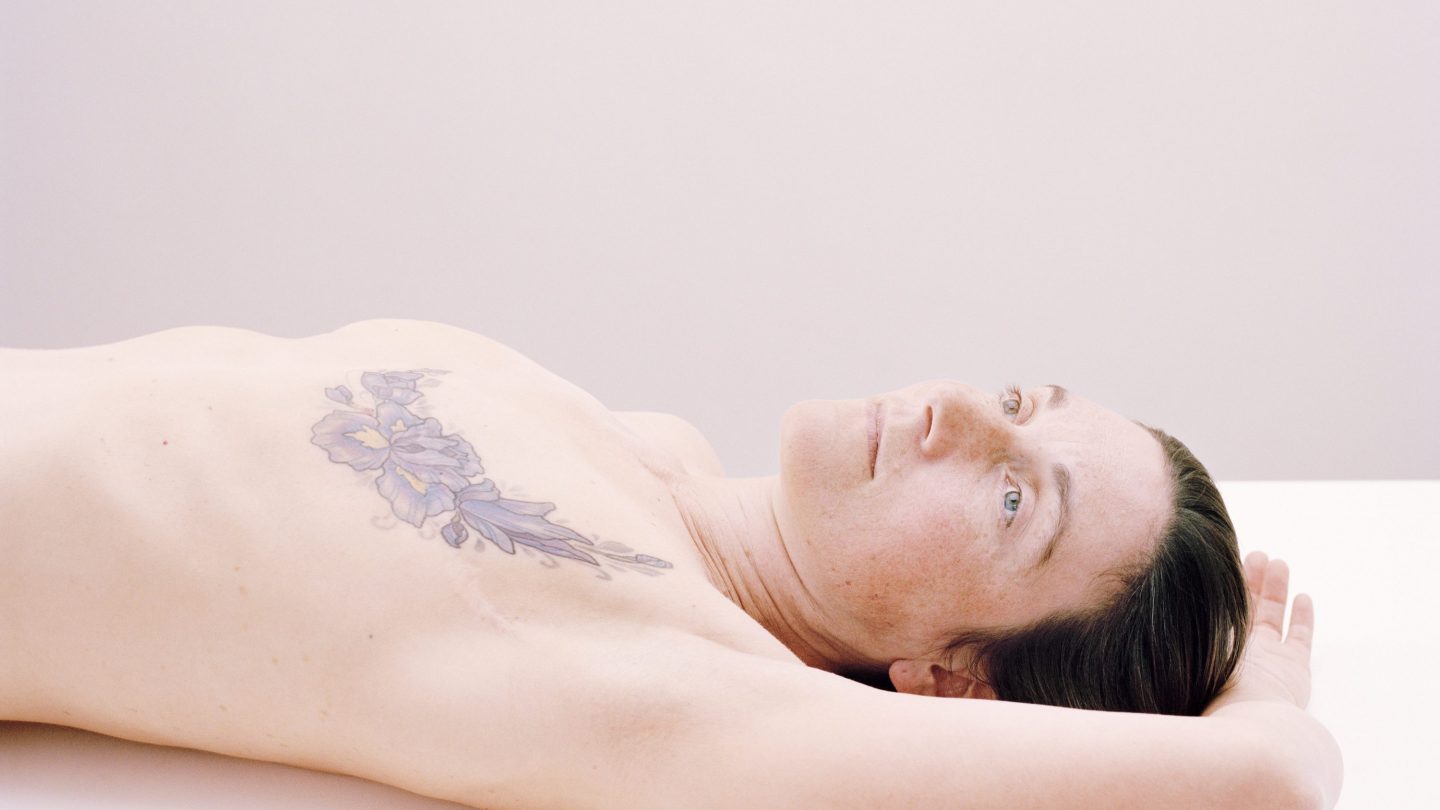
Reclaim
This series of portraits, by photographer Kate Peters, explores the ideas of alternative beauty and power by photographing breast cancer survivors who have transformed their scars with tattoos. This powerful act is a way in which women can reclaim their bodies from breast cancer and strengthen their personal identity in the process.
Read more about Cambridge’s efforts to cure cancer and how you can support it.